NOTICE
ANSM - Last updated: 06/11/2013
Name of the medicinal product
MYLEUGYNE 1% cream
Econazole Nitrate
framed
Read this leaflet carefully before you start using this medicine. It contains important information for your treatment.
If you have any further questions, ask your doctor or pharmacist.
· Keep this leaflet, you may need to read it again.
· If you need more information and advice, ask your pharmacist.
· If symptoms worsen or persist, consult your doctor.
· If you notice any side effects not listed in this leaflet, or if you experience any of the effects listed as serious, please tell your doctor or pharmacist.
Review summary
In this notice :
1. WHAT IS MYLEUGYNE 1%, cream AND WHAT IT IS USED FOR?
2. BEFORE YOU USE MYLEUGYNE 1%, cream?
3. HOW TO USE MYLEUGYNE 1%, cream?
4. WHAT ARE POSSIBLE SIDE EFFECTS?
5. HOW TO STORE MYLEUGYNE 1%, cream?
6. ADDITIONAL INFORMATION
1. WHAT IS MYLEUGYNE 1%, cream AND WHAT IT IS USED FOR?
Pharmacotherapeutic group
IMIDAZOLE DERIVATIVES
LOCAL ANTIFUNG
(D: Dermatology)
Therapeutic indications
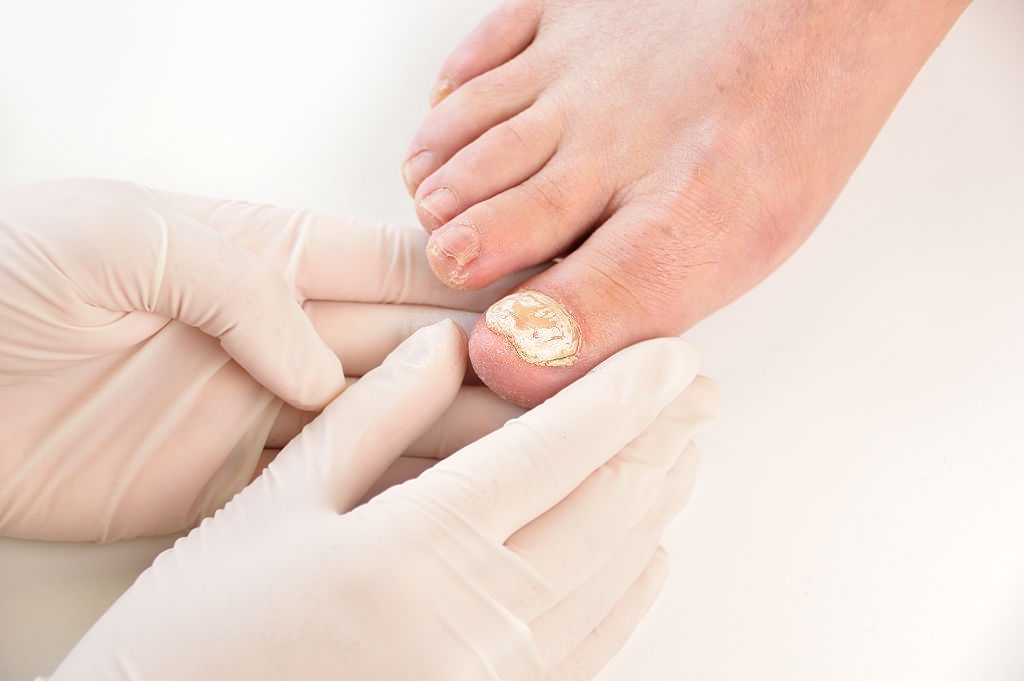
This medication is recommended for the treatment or adjunctive treatment of certain cutaneous diseases caused by fungi (mycoses).
2. BEFORE YOU USE MYLEUGYNE 1%, cream?
List of information needed before taking the medication
Not applicable.
Cons-indications
Never use MYLEUGYNE 1%, cream if you are allergic to any of the ingredients.
IN CASE OF DOUBT, IT IS ESSENTIAL TO ASK FOR THE OPINION OF YOUR DOCTOR OR YOUR PHARMACIST.
Precautions for use; special warnings
Take special care with MYLEUGYNE 1%, cream:
Precautions for use
· Avoid application near eyes.
· It is recommended not to use an acid soap (acidity favoring the multiplication of candida).
· In case of application in children, on a large surface or on injured skin, it is imperative that you observe the recommendations and the dosage indicated by your doctor because of the greater penetration of the product in these circumstances.
Due to the presence of benzoic acid, this medicinal product may cause irritation to the skin, eyes and mucous membranes.
This medicinal product contains butylhydroxyanisole (E320) and butylhydroxytoluene (E321) and may cause local skin reactions (eczema) or irritation of the eyes and mucous membranes.
IN CASE OF DOUBT DO NOT HESITATE TO REQUEST THE NOTICE OF YOUR DOCTOR OR PHARMACIST.
Interaction with other medicines
Taking or using other medicines
If you are taking or have recently taken any other medicines, including medicines obtained without a prescription, talk to your doctor or pharmacist.
Interactions with food and beverages
Not applicable.
Interactions with Herbal Medicines or Alternative Therapies
Not applicable.
Use during pregnancy and lactation
Not applicable.
Sport
Not applicable.
Effects on ability to drive or use machines
Not applicable.
List of excipients with known effect
List of excipients with known effect: benzoic acid, butylhydroxyanisole, butylhydroxytoluene.
3. HOW TO USE MYLEUGYNE 1%, cream?
Instructions for proper use
Not applicable.
Dosage, Mode and / or route (s) of administration, Frequency of administration and Duration of treatment
Dosage
Apply to the affected areas and their periphery 2 times a day, after washing and drying the skin. Follow the application of a gentle and regular massage until complete penetration.
Administration mode
DERMAL
Duration of the treatment
The duration of treatment is 2 to 4 weeks depending on the mycosis, it may be longer for some localizations. Regular use of the product throughout the course of treatment is critical to successful treatment.
Symptoms and Instructions for Overdose
Not applicable.
Instructions for omission of one or more doses
Not applicable.
Risk of withdrawal syndrome
Not applicable.
4. WHAT ARE POSSIBLE SIDE EFFECTS?
Description of adverse reactions
Like all medicines, MYLEUGYNE 1%, cream is likely to have unwanted effects, although not everyone is subject to it:
Itching, irritation, burning sensation, redness and in very rare cases, allergic reactions with eczema type have been observed: IT MUST BE TESTED BY YOUR DOCTOR.
If you notice any side effects not listed in this leaflet, or if any of the side effects gets serious, contact your doctor or pharmacist.
5. HOW TO STORE MYLEUGYNE 1%, cream?
Keep out of sight and reach of children.
Expiration date
Do not use MYLEUGYNE 1%, cream after the expiry date which is stated on the carton. The expiry date refers to the last day of the month.
Storage conditions
Store at a temperature not exceeding 25 ° C.
If necessary, warnings against visible signs of deterioration
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist what to do with unused medications. These measures will help protect the environment.
6. ADDITIONAL INFORMATION
Full list of active substances and excipients
What contains MYLEUGYNE 1%, cream?
The active substance is:
Econazole Nitrate .............................................. .................................................. ................................. 1 g
For 100 g of cream.
The other components are:
Palmitostearate of ethylene glycol and polyoxyethylene glycols (TEFOSE 63), oleic macrogolglycerides (LABRAFIL M 1944 CS), light liquid paraffin, benzoic acid, butylhydroxyanisole (E320), perfume (essential oils of lavandin, orange and mandarin, linalyl acetate , citronellol, butylhydroxytoluene (E321), dipropylene glycol), purified water.
Pharmaceutical form and content
What is MYLEUGYNE 1%, cream and contents of the pack?
This medication is in the form of a cream. Tube of 30 g.
Name and address of the marketing authorization holder and the holder of the manufacturing authorization responsible for the release of the lots, if different
Holder
IPRAD PHARMA LABORATORIES
174 quai de Jemmapes
75010 PARIS
exploiting
IPRAD PHARMA LABORATORIES
174 quai de Jemmapes
75010 PARIS
Maker
CHEMINEAU LABORATORIES
93 ROAD OF CURRENCY
37210 VOUVRAY
Names of the medicinal product in the Member States of the European Economic Area
Not applicable.
Date of approval of the notice
The last date on which this leaflet was approved is {date}.
AMM under exceptional circumstances
Not applicable.
Internet Information
Detailed information on this medicine is available on the Ansm website (France) www.ansm.fr.
Information for health professionals only
Not applicable.
Other
Not applicable.