NOTICE
ANSM - Updated: 23/03/2018
Name of the drug
GYNOPURA 1%, cream for local application
Econazole nitrate
framed
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
· Keep this leaflet. You might need to read it again.
· If you have further questions, ask your doctor or pharmacist.
· This medicine has been prescribed for you. Do not give this to anyone else. It could be harmful to them, even if the signs of their illness are the same as yours.
· If you get any side effects, talk to your doctor or pharmacist. This also applies to any undesirable effect that is not mentioned in this leaflet. See section 4.
What does this booklet contain ?
1. What is GYNOPURA 1%, cream for local application and in which cases is it used?
2. What information do you need to know before using GYNOPURA 1% cream for local application?
3. How to use GYNOPURA 1%, cream for local application?
4. What are the possible side effects?
5. How to store GYNOPURA 1%, cream for local application?
6. Contents of the package and other information.
1. WHAT GYNOPURA 1%, cream for local application AND WHAT IT IS USED FOR
Pharmacotherapeutic group - ATC code: Not applicable
IMIDAZOLE DERIVATIVES
LOCAL ANTIFUNGAL
(D: Dermatology)
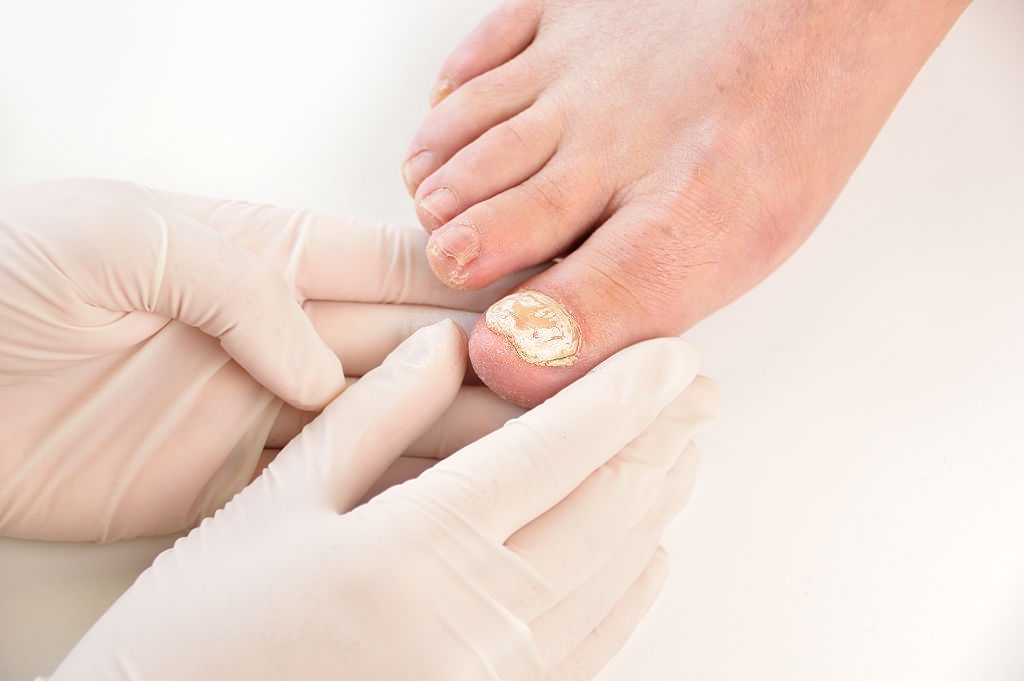
This medication is recommended for the treatment or adjunctive treatment of certain skin conditions caused by fungi (fungal infections).
2. WHAT YOU NEED TO KNOW BEFORE YOU USE GYNOPURA 1%, local application cream?
Never use GYNOPURA 1%, cream for local application
· if you are allergic to the active substance} or to any of the other ingredients of this medicine, mentioned in section 6.
IN CASE OF DOUBT, IT IS ESSENTIAL TO ASK THE ADVICE OF YOUR DOCTOR OR PHARMACIST.
Warnings and precautions
Be careful with GYNOPURA 1%, cream for local application:
Precautions for use
· Avoid application near the eyes.
· It is recommended not to use an acidic soap (the acidity favoring the multiplication of candida).
· If used in children, on a large surface, or on an injured skin, it is imperative to respect the recommendations and the dosage indicated by your doctor because of the greater penetration of the product in these circumstances.
IF IN DOUBT, DO NOT HESITATE TO ASK YOUR DOCTOR OR PHARMACIST FOR ADVICE.
Children and adolescents
Never leave within the reach of children.
Other medicines and GYNOPURA 1%, cream for local application
If you are taking or have recently taken any other medicines, including medicines obtained without a prescription, tell your doctor or pharmacist.
GYNOPURA 1%, cream for local application with food and drinks
Not applicable.
Pregnancy and breast feeding
Seek advice from your doctor or pharmacist before taking any medicine.
Driving and using machines
Not applicable.
GYNOPURA 1%, local application cream contains
Not applicable.
3. HOW TO USE GYNPOPURA 1%, cream for local application?
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if in doubt.
Drug for local use:
Apply on affected areas and their periphery 2 times a day, after washing and drying the skin. Follow the application of a gentle and regular massage until complete penetration.
DERMAL
The duration of treatment is 2 to 4 weeks depending on the mycosis, it can be longer for some localizations. Regular use of the product throughout the course of treatment is critical to successful treatment.
IN ALL CASES, COMPLY STRICTLY WITH THE ORDER OF YOUR DOCTOR.
If you use more GYNOPURA 1%, local application cream than you should
Not applicable.
If you forget to use GYNOPURA 1%, cream for local application
Not applicable.
If you stop using
Not applicable.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist for more information.
4. WHAT ARE THE POSSIBLE SIDE EFFECTS?
· Like all medicines, GYNOPURA 1%, local application cream is likely to have side effects, although not everybody gets them:
· Itching, irritation, burning sensation, redness and in very rare cases allergic reactions such as eczema have been observed: WARNING YOUR DOCTOR.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This also applies to any undesirable effect that is not mentioned in this leaflet. You can also report side effects directly via the national reporting system: National Agency for the Safety of Medicines and Health Products (ANSM) and the network of Regional Pharmacovigilance Centers - Website: www.ansm.sante.fr
By reporting side effects, you can help provide more information about the safety of the medicine.
5. HOW TO STORE GYNOPURA 1%, cream for local application?
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton.
Do not dispose of any medication in the sewage system or in the household garbage. Ask your pharmacist to eliminate medications that you no longer use. These measures will help protect the environment.
6. CONTENTS OF PACKAGING AND OTHER INFORMATION
What GYNOPURA 1% contains, cream for local application
· The active substance is:
Econazole nitrate .............................................. .................................................. .............. 1 g
For 100 g of cream
· The other components are:
Palmitostearate of ethylene glycol and polyoxyethylene glycols (TEFOSE 63), unsaturated polyglycolysed glycerides (LABRAFIL M 1944 CS), light liquid paraffin, benzoic acid, butylhydroxyanisole, perfume PVC 1676 (essential oils of lavandin, orange and mandarin, linalyl acetate citronellol, butylhydroxytoluene, dipropylene glycol), purified water.
What GYNOPURA 1%, cream for topical application and contents of the pack
This medicine comes in the form of cream. Box of 1 tube (30 g).
Marketing Authorization Holder
BESINS INTERNATIONAL LABORATORIES
3 RUE DU BOURG THE ABBE
75003 PARIS
Operator of the marketing authorization
BESINS INTERNATIONAL LABORATORIES
13 PERIER STREET
92120 MONTROUGE
Maker
THEPENIER PHARMA & COSMETICS
DEPARTMENTAL ROUTE 92
61400 SAINT LANGIS LES MORTAGNE
OR
BESINS INTERNATIONAL LABORATORIES
13 PERIER STREET
92120 MONTROUGE
Names of the drug in the member states of the European Economic Area
Not applicable.
The last date this leaflet was revised is:
[to be completed later by the holder]
<{MM / YYYY}> <{YYYY month}.>
Other
Detailed information on this medicine is available on the ANSM website (France).