NOTICE
ANSM - Last updated: 17/08/2017
Name of the medicinal product
ARCALION 200mg, coated tablet
Sulbutiamine
framed
Please read this leaflet carefully before you start taking this medicine because it contains important information for you.
You should always take this medication exactly as prescribed in this leaflet or by your doctor, pharmacist or nurse.
· Keep this leaflet. You might need to read it again.
· Ask your pharmacist for advice or information.
· If you experience any side effects, talk to your doctor, pharmacist or nurse. This also applies to any side effects not mentioned in this leaflet. See section 4.
· You should contact your doctor if you experience no improvement or feel less well after 4 weeks.
What is in this leaflet?
1. What is ARCALION 200 mg , coated tablet and in which cases it is used?
2. What you need to know before taking ARCALION 200 mg coated tablet?
3. How to take ARCALION 200 mg coated tablet ?
4. What are the possible side effects?
5. How to store ARCALION 200 mg coated tablet ?
6. Package contents and other information.
1. WHAT ARCALION, 200 mg, coated tablet AND IN WHAT CASES IS IT USED?
Pharmacotherapeutic group: Vitamin B1, unassociated - ATC code: A11DA02
This drug is indicated in some states of adult fatigue (more than 15 years).
You should contact your doctor if you experience no improvement or feel less well after 4 weeks.
2. BEFORE YOU TAKE ARCALION, 200 mg, coated tablet?
If your doctor has told you about an intolerance to some sugars, contact your doctor before taking this medicine.
Never take ARCALION 200 mg, coated tablet:
· if you are allergic to sulbutiamine or any of the other ingredients of this medication mentioned in section 6.
Warnings and Precautions
This medicine contains glucose, lactose and sucrose. The use of this medication is not recommended in patients with glucose and / or galactose malabsorption syndrome (rare hereditary diseases), galactose intolerance, Lapp lactase deficiency or sucrase / isomaltase (rare hereditary diseases), or fructose intolerance.
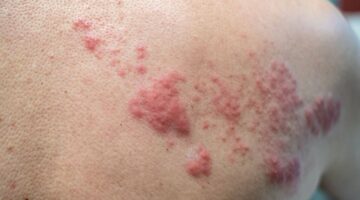
This medicinal product contains an azo coloring agent, orange yellow S (E110) and may cause allergic reactions (see section 4. "What are the possible adverse effects?").
If symptoms persist for more than 4 weeks, consult your doctor.
Talk to your doctor, pharmacist or nurse before taking ARCALION 200 mg coated tablet.
Children and Youth
ARCALION should not be used in children and adolescents.
Other medicines and ARCALION 200 mg coated tablet
Inform your doctor or pharmacist if you are taking, have recently taken or may take any other medicines. ARCALION can interact with the following medications:
· diuretics (used in the treatment of high blood pressure) may increase urinary excretion of thiamine (vitamin B1, derived from sulbutiamine).
· Neuro-muscular blocking agents (used in general anesthesia) whose effects can be increased with thiamine (vitamin B1, derived from sulbutiamine).
ARCALION 200 mg, coated tablet, with food, beverages and alcohol
Not applicable.
Pregnancy, breast-feeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or plan a pregnancy, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
As a precaution, it is preferable to avoid the use of ARCALION during pregnancy. Tell your doctor if you think you are pregnant.
feeding
You should not take ARCALION if you are breast-feeding. Tell your doctor immediately if you are breast-feeding or are about to start breast-feeding.
Driving and using machines
Not applicable.
ARCALION 200 mg, coated tablet contains: glucose, lactose, orange yellow lacquer S (E 110) and sucrose.
3. HOW TO TAKE ARCALION, 200 mg, coated tablet?
Always take this medication exactly as prescribed by your doctor or pharmacist. Check with your doctor or pharmacist if in doubt.
Dosage
RESERVED FOR ADULTS
2 to 3 tablets per day.
The tablets should be swallowed as they are with a large glass of water, spreading the catch to the morning and midday meals.
Treatment time limited to 4 weeks.
Always take this medication exactly as prescribed in this leaflet or as directed by your doctor, pharmacist or nurse. Check with your doctor, pharmacist or nurse if in doubt.
Administration mode
Oral use.
Duration of treatment
Do not use more than 4 weeks.
If you take more ARCALION 200 mg, coated tablet than you should
In case of overdose, symptoms may include agitation and tremors of the extremities.
Immediately consult your doctor or pharmacist
If you forget to take ARCALION 200 mg, coated tablet
Not applicable.
If you stop taking ARCALION 200 mg, coated tablet
Not applicable.
If you have any further questions on the use of this medication, ask your doctor, pharmacist or nurse for more information.
4. WHAT ARE POSSIBLE SIDE EFFECTS?
Like all medicines, this medicine may cause side effects, although not everybody gets them.
The following adverse reactions were observed:
Uncommon (occurred in less than 1 user in 100 but in more than 1 user in 1000)
· skin rash,
· want to vomit (nausea), vomiting,
· agitation,
· headache,
· tremors,
· discomfort.
Frequency not known (can not be estimated from available data)
· pain in the stomach, diarrhea.
Due to the presence of orange-yellow S, there is a risk of allergic reactions.
Declaration of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse. This also applies to any side effects not mentioned in this leaflet. You can also report adverse reactions directly via the national reporting system: National Agency for the Safety of Medicines and Health Products (ANSM) and network of Regional Centers of Pharmacovigilance - Website: www.ansm.sante.fr
By reporting adverse reactions, you are helping to provide more information about the safety of the drug.
5. HOW TO STORE ARCALION 200 mg coated tablet?
Keep this medicine out of the reach and sight of children.
Do not use this medicine after the expiry date which is stated on the package. The expiry date refers to the last day of that month.
Blister pack: The tablets should be stored at a temperature not exceeding 25 ° C.
Pill: The tablets should be stored at a temperature not exceeding 30 ° C.
Do not throw any medicines into drains or rubbish. Ask your pharmacist to remove any medications you are no longer using. These measures will help protect the environment.
6. PACKAGE CONTENTS AND OTHER INFORMATION
What ARCALION 200 mg contains: coated tablet
· The active substance is:
Sulbutiamine ....................................... .............................. ....................................... 200 mg
For a coated tablet
· The other components are:
Starch starch, anhydrous glucose, lactose monohydrate, magnesium stearate, talc, sodium bicarbonate, sodium carmellose, white beeswax, titanium dioxide (E171), ethylcellulose, E 110), glycerol monooleate, polysorbate 80, povidone, sucrose, anhydrous colloidal silica (Aerosil 130).
What is ARCALION 200 mg, coated tablet and contents of the pack
This medication is in the form of a coated tablet. Box of 15, 20, 30, 60 or 100 coated tablets.
Not all pack sizes may be marketed.
Marketing Authorization Holder
SERVIER LABORATORIES
50, RUE CARNOT
92284 SURESNES CEDEX
LA FRANCE
Marketing Authorization Operator
SERVIER LABORATORIES
50, rue Carnot
92284 SURESNES Cedex
LA FRANCE
Maker
LABORATOIRES SERVIER INDUSTRIE
905 route de Saran
45520 GIDY
LA FRANCE
Names of the medicinal product in the Member States of the European Economic Area
Not applicable.
The last date on which this leaflet was revised is:
{MM / YYYY} {YYYY months}.
Other
Detailed information on this medicine is available on the ANSM website (France).