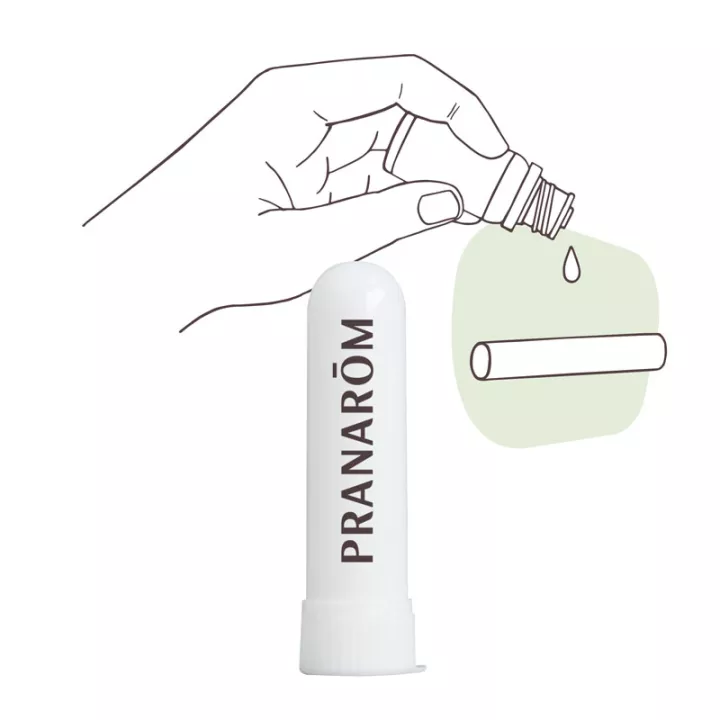
HUMER Inhaler Respiratory discomfort
Decongests the upper airways Thanks to the decongestant action ofeucalyptus globulus essential oil, HUMEX INHALER gives you a feeling of freshness.
Description of HUMER Inhaler Respiratory discomfort
This medical device is used as a decongestant for common respiratory ailments(colds, rhinitis, rhinopharyngitis) in adults and children over 6 years of age. Humer inhaler decongests the upper airways thanks to the camphor and eucalyptus essential oils it contains, giving you an immediate sensation of freshness and respiratory comfort.
Directions for use and dosage
For adults and children over 6 years of age.
2 inhalations per nostril per dose, repeated if necessary, up to a maximum of 3 doses per day, spread evenly throughout the day.
If symptoms persist beyond 3 days or worsen, consult your doctor.
Give your opinion on the advice for use and dosage of HUMER Inhaleur Inconfort respiratoire with our partner Verified opinions after your purchase.
Composition
Active ingredients: 99.85%eucalyptus globulusessential oil, 100% natural origin, 0.15% camphor.
Free Your Airways
-
Decongestant action: HUMER INHALER is specially formulated to provide effective decongestion of the upper airways. Thanks to eucalyptus globulus essential oil, this inhaler provides a soothing sensation of freshness.
-
Symptom relief: If you suffer from nasal congestion, a blocked nose or breathing difficulties due to congestion, HUMER INHALER can bring you immediate relief.
-
Breathing comfort: By clearing your airways, this inhaler helps you breathe more easily and regain optimum breathing comfort, whether you're an adult or a child over 6.
Your Solution for Clear Breathing
-
Refreshing effect: Eucalyptus Globulus essential oil is renowned for its refreshing and decongestant properties. It provides a pleasant sensation of freshness when inhaled.
-
Easy to use: HUMER INHALER comes in a practical inhaler that's easy to take with you. You can use it whenever you need relief.
-
For the whole family: Whether you're an adult or have a child over 6, HUMER INHALER is suitable for the whole family for clear breathing and immediate well-being.
Breathe Deeply and Freely
Make HUMER INHALER your ally for clear airways and free breathing. Enjoy the soothing freshness of eucalyptus globulus essential oil with every inhalation.
Precautions for use
- In the event of an allergic reaction, discontinue use.
- If symptoms persist for more than 3 days and/or signs of superinfection appear, in the event of fever, purulent nasal discharge or purulent sputum, consult your doctor.
- Do not use if pregnant or breast-feeding.
-
Read this leaflet carefully, as it contains important information.
-
If symptoms persist, worsen or develop, seek medical advice.
Presentation of HUMER Inhaler Respiratory discomfort
Available in Box 1 inhaler tube.
Our advice and expert opinions in pharmacy
Nasal congestion (blocked nose) is caused by local inflammation or irritation, which tightens the passage of air through the nose.
When accompanied by a cold, sinusitis (inflammation of the sinuses, cavities in certain facial bones where the mucous membranes that humidify the air are found), allergic rhinitis or mechanical blockage, nasal congestion may indicate the presence of an infection, allergy or nasal obstruction.