Description of Dépuratum Lehning indicated for drainage and detoxification
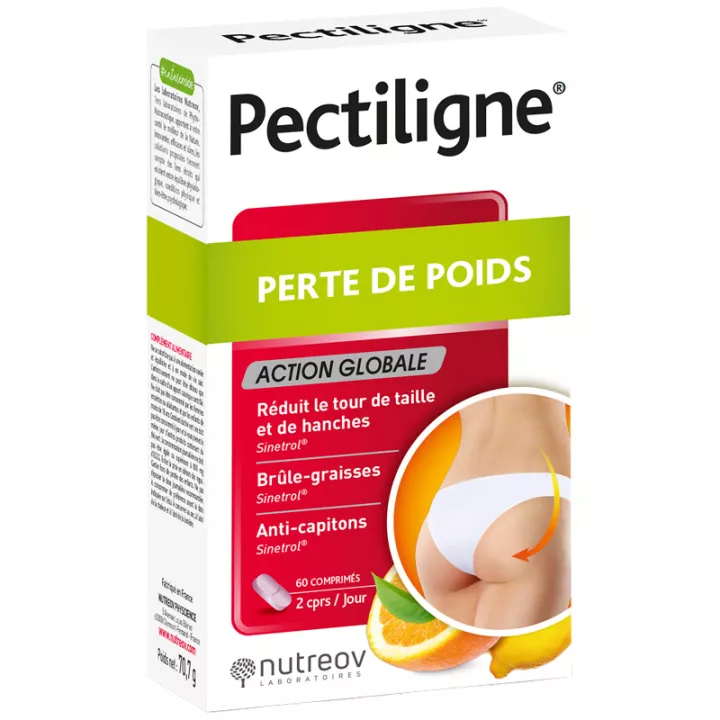
Dépuratum: a synergy of 7 plants for harmonious detoxification
Dépuratum by Lehning Laboratories is a dietary supplement designed to support the body's natural drainage and detoxification processes. Formulated from a unique combination of 7 plants carefully selected for their beneficial properties, Dépuratum promotes toxin elimination and contributes to general well-being. With juniper, wild rhubarb, thyme, prickly pear cactus, birch, rosemary and fumitory, this dietary supplement is a true gift of nature for those seeking to revitalize their bodies in a gentle, natural way.
Natural support for thedigestive and liver systems
The selection of plants in Dépuratum Lehning specifically targetsthe digestive and hepatic systems, helping to stimulate elimination functions and support the liver in its crucial detoxification role. By facilitating digestion and promoting the elimination of unwanted substances, Dépuratum helps purify the body, promoting a feeling of lightness and renewed energy.
A detox cure for everyone
Whether you'd like to start a detox to counter a feeling of heaviness, after overeating or simply to keep your body in good health, Dépuratum is tailored to your needs. Easy to integrate into your daily routine, this dietary supplement is the ideal companion for those who aspire to a healthy lifestyle, in harmony with their body's natural cycles.
What is Lehning's Depuratum food supplement used for?
After the festive season or as the summer approaches, the body needs toeliminate excesses, and the liver needs to rest. For your detox cures, discover Dépuratum and its unique formula combining 7 plants known to detoxify, promote digestive comfort and aid hepatobiliary drainage.
- Juniper, wild rhubarb, thyme and prickly pear cactus promote digestive comfort.
- Birch and rosemary aid detoxification.
- Fumitory aids hepatobiliary drainage.
How to use this dietary supplement for drainage and detoxification?
Take 1 capsule two or three times a day with a full glass of water, preferably before meals.
How should I take Lehning Depuratum?
Follow the dosage recommendations provided with the product, or consult a healthcare professional to adapt the treatment to your specific needs.
Is Dépuratum suitable for everyone?
Dépuratum is designed for adults seeking natural support for detoxification. However, if you are pregnant or breastfeeding, or if you have any special medical conditions, we recommend that you consult a healthcare professional before starting the treatment.
How long does a Depuratum cure last?
The duration of a Depuratum cure may vary according to individual objectives and the advice of a healthcare professional. It is important to follow the instructions provided and not to exceed the recommended duration without medical advice.
Are there any side effects?
Dépuratum is formulated to be well tolerated. However, as with any dietary supplement, watch for any unusual reaction and consult a healthcare professional if in doubt.
What are the precautions for use?
- Adults and children over 12 years of age.
- Avoid in case of hypersensitivity to any of the ingredients.
- Do not exceed recommended daily dose.
- Keep out of reach of children.
- Not recommended in cases of allergy to salicylates or for women with a personal or family history of breast cancer.
- Do not use for more than 6 weeks in cases of renal insufficiency, anticoagulant treatment, pregnancy or breast-feeding.
- Keep out of reach of children.
Composition of this food supplement Dépuratum by Lehning
- Ingredients :
- Juniper (fruit) 24%, Wild rhubarb (root and rhizome) 24%, Birch (leaf) 12%, Rosemary (leaf) 12%, Thyme (flowering top) 9%, Fumitory (aerial part) 9%, Beef stopper (root) 7%, Magnesium stearate (anti-caking agent),
- Capsule shell :
- gelatin, caramel colorant.
- Nutritional values (Quantity of active ingredients per 3 capsules) :
- Juniperus communis (Juniper): 306 mg
- Rheum rhaponticum (Wild Rhubarb): 306 mg
- Thymus vulgaris (Thyme): 114.75 mg
- Ononis spinosa (Beef stopper): 89.25 mg
- Betula pendula (Birch): 153 mg
- Rosmarinus officinalis (Rosemary): 153 mg
- Fumaria officinalis (Fumitory): 14.75 mg
Packaging
1 box containing 60 capsules with 7 plants.