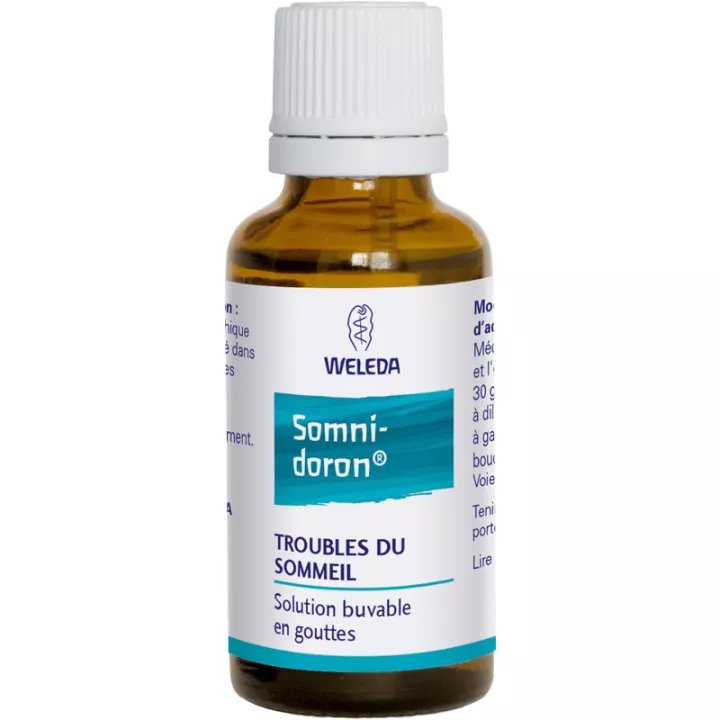
What is Weleda Somnidoron Oral Solution Drops 30 ml used for?
Sleep disorders are a common problem that has a considerable impact on quality of life. The inability to fall asleep quickly, frequent night-time awakenings or poor-quality sleep can lead to chronic fatigue, irritability and reduced cognitive performance. Weleda Somnidoron is a homeopathic drinkable solution specifically formulated to promote natural, soothed sleep.
A homeopathic complex to rebalance sleep
Weleda Somnidoron is based on a synergistic formulation of three homeopathic active ingredients known for their gentle, soothing sedative effects on the nervous system:
- Coffea Tosta 20DH: Derived from roasted coffee beans, this active ingredient is traditionally used to alleviatecerebral hyperactivity and reduce intrusive thoughts that prevent sleep.
- Stramonium 12DH: Present in dilution, it acts onnocturnal anxiety, sudden awakenings and states of agitation that disrupt sleep continuity.
- Valeriana Officinalis 3DH: A medicinal plant renowned for its relaxing properties, it promotes relaxation of the nervous system and improves sleep quality.
These active ingredients, combined according to the principles of homeopathy, enable a progressive and respectful approach to the natural sleep cycle, without causing daytime drowsiness or undesirable side effects.
Effective action on minor sleep disorders
Weleda Somnidoron is designed to support individuals suffering from sleep difficulties linked to stress, mental preoccupation or excessive excitement at the end of the day. Thanks to its modulating action on the central nervous system, this solution helps to :
- Reduce the time it takes to fall asleep by reducing mental hyperactivity.
- Prolong sleep duration by minimizing night-time awakenings.
- Improve the quality of night-time rest by inducing a state of deep relaxation.
Its natural mode of action makes it an interesting alternative to heavier treatments, such as benzodiazepines or sedative antihistamines, which can induce habituation and residual effects upon awakening.
A solution adapted to modern lifestyles
Many modern factors disrupt sleep quality: cognitive overload, prolonged exposure to screens, unsuitable diet or staggered working hours. Weleda Somnidoron can be easily integrated into a daily routine thanks to :
- Simple to take in drops, to be diluted in a little water.
- <>A natural formulation that respects the body's biological balance.
- A gentle, gradual effect, without causing addiction or dependency.
Restorative sleep without side effects
Unlike conventional sleeping pills, which can alter sleep patterns by reducing the REM sleep phase, Weleda Somnidoron acts physiologically to optimize night-time recovery. Its formula allows you to wake up without feeling heavy, and preserves daytime cognitive functions.
By acting on both the nervous system and emotional balance, this product offers a holistic, natural approach to regulating sleep disorders and restoring a harmonious night-time rhythm.
A product recommended for all-round care
To maximize its effectiveness, Weleda Somnidoron can be combined with an adapted sleep hygiene program: reduction of light sources before bedtime, regular sleep schedules and stress management during the day. In addition, its use can be reinforced by Valeriana Officinalis Boiron, available in granules, doses or drinkable solution, for a complementary action on relaxation and falling asleep.
Instructions for use
Weleda Somnidoron is taken orally, diluted in a little water. Before swallowing, keep the solution in the mouth for a few moments for better absorption.
- Dosage: Take 30 drops at bedtime, diluted in a little water.
- First use: Unscrew cap completely to break off tamper-evident ring.
- Dosage: Take the recommended number of drops using the built-in dropper.
- Duration of treatment: Do not exceed 15 days without medical advice.
Give your opinion on the use and dosage of Weleda Somnidoron with our partner Avis Vérifiés after your purchase.
Precautions for use
- For adults and children over 12 years of age only.
- Do not use on children under 12.
- Contains ethanol (alcohol) : 369 mg per dose of 30 drops.
- Do not exceed recommended dose.
- If in doubt about dosage, seek advice from a healthcare professional.
- Pregnant and breast-feeding women : Use is possible, but it is recommended to consult a doctor before starting treatment.
What does it contain?
Coffea Tosta 20Dh, Stramonium 12Dh, Valeriana Officinalis 3Dh, Ethanol, Purified water.
Presentation
Weleda Somnidoron is available in a 30 ml bottle, with a practical dropper for easy use and precise dosage.