NOTICE
ANSM - Updated on: 09/01/2009
Name of the medicinal product
ECONAZOLE MYLAN 1%, solution for skin application
framed
Read this leaflet carefully before you start taking this medicine. It contains important information for your treatment.
· If you have any further questions, ask your doctor or pharmacist.
· Keep this leaflet, you may need to read it again.
If you need more information and advice, ask your pharmacist.
If symptoms worsen or persist, consult a physician.
Review summary
In this notice :
1. WHAT IS ECONAZOLE MYLAN 1%, solution for dermal application AND WHAT IT IS USED FOR?
2. BEFORE YOU USE ECONAZOLE MYLAN 1% solution for skin application?
3. HOW TO USE ECONAZOLE MYLAN 1%, solution for skin application?
4. WHAT ARE POSSIBLE SIDE EFFECTS?
5. HOW TO STORE ECONAZOLE MYLAN 1% solution for skin application?
6. ADDITIONAL INFORMATION
1. WHAT IS ECONAZOLE MYLAN 1%, solution for dermal application AND WHAT IT IS USED FOR?
Pharmacotherapeutic group
This medication is an antifungal for topical use.
Therapeutic indications
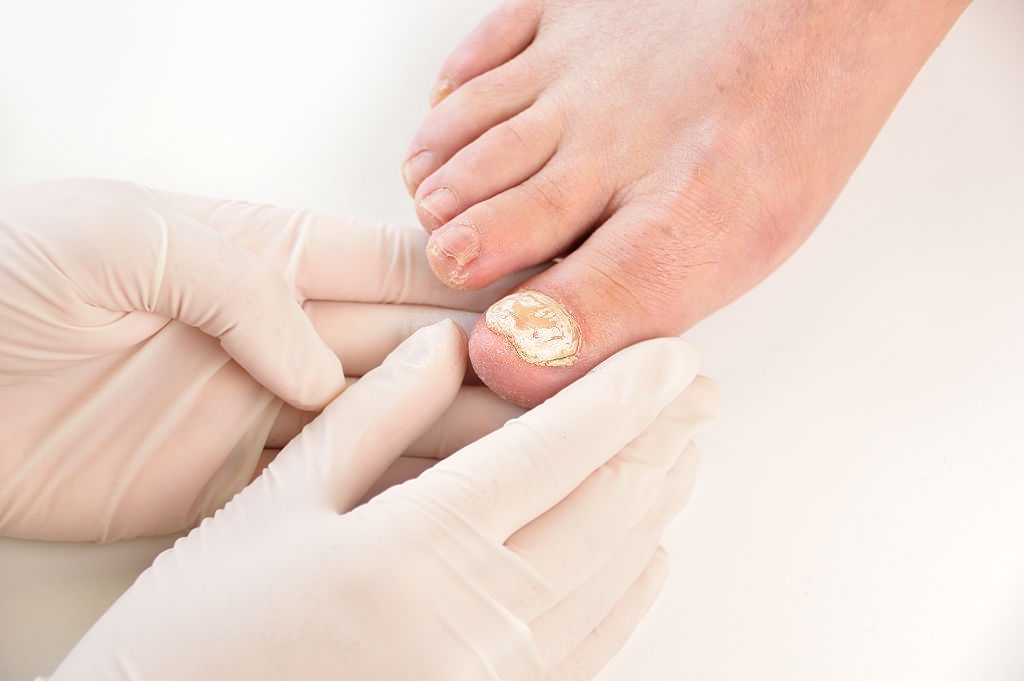
It is used in the treatment or adjunctive treatment of certain cutaneous diseases due to fungi (mycoses).
2. BEFORE YOU USE ECONAZOLE MYLAN 1% solution for skin application?
List of information needed before taking the medication
Not applicable.
Cons-indications
Do not use ECONAZOLE MYLAN 1% solution for skin application in the following cases:
Allergy known to econazole, related substances (imidazole) or any of the other ingredients of this medicine.
Precautions for use; special warnings
Avoid application near the eyes and on the genital mucous membranes.
It is recommended not to use acid soap (the acidity favoring the multiplication of Candida).
In case of application in children, on a large surface, or injured skin, it is imperative that you observe the recommendations and the dosage indicated by your doctor due to the greater penetration of the product in these circumstances.
Interaction with other medicines
Taking or using other medicines:
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, even medicines obtained without a prescription.
Interactions with food and beverages
Not applicable.
Interactions with Herbal Medicines or Alternative Therapies
Not applicable.
Use during pregnancy and lactation
Pregnancy - Breastfeeding
Ask your doctor or pharmacist for advice before taking any medicine.
Sport
Not applicable.
Effects on ability to drive or use machines
Not applicable.
List of excipients with known effect
List of excipients with known effect: butylhydroxytoluene (E321), ethanol, propylene glycol.
3. HOW TO USE ECONAZOLE MYLAN 1%, solution for skin application?
Instructions for proper use
Not applicable.
Dosage, Mode and / or route (s) of administration, Frequency of administration and Duration of treatment
Dermal.
Apply to the affected areas and their periphery, twice a day, after washing and drying the skin.
Follow the application of a gentle and regular massage until complete penetration.
Shake the bottle before use.
Close the bottle after each use.
If you feel that the effect of ECONAZOLE MYLAN 1%, solution for skin application is too strong or too weak, consult your doctor or pharmacist.
The duration of the treatment is 2 to 4 weeks depending on the mycosis, it can be longer for certain localizations (nails).
Regular use of the product throughout the course of treatment is critical to successful treatment.
Symptoms and Instructions for Overdose
If you have used more ECONAZOLE MYLAN 1% solution for skin application than you should: consult your doctor or pharmacist.
Instructions for omission of one or more doses
If you forget to use ECONAZOLE MYLAN 1%, solution for skin application: do not use a double dose to compensate for the single dose you forgot to use.
Risk of withdrawal syndrome
Not applicable.
4. WHAT ARE POSSIBLE SIDE EFFECTS?
Description of adverse reactions
Like all medicines, ECONAZOLE MYLAN 1%, solution for skin application is usable to have undesirable effects:
· Itching, irritation, burning sensation, redness and in very rare cases, allergic reactions with eczema type have been observed: IT MUST BE TESTED TO YOUR DOCTOR.
· Frequent applications on the skin can cause irritation and dryness of the skin, due to the presence of ethanol,
· Risk of eczema (allergic skin reaction), irritation of the skin and mucous membranes due to the presence of propylene glycol and butylhydroxytoluene (E321).
In these cases, tell your doctor.
If you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE ECONAZOLE MYLAN 1% solution for skin application?
Keep out of the reach and sight of children.
Expiration date
Do not use after the expiry date stated on the vial, box.
Storage conditions
Pressurized container:
· Do not expose to excessive heat,
· Do not drill,
· Do not throw into the fire even empty.
If necessary, warnings against visible signs of deterioration
Not applicable.
6. ADDITIONAL INFORMATION
Full list of active substances and excipients
The active substance is :
Econazole Nitrate .............................................. .................................................. ................................. 1 g
For 100 g of solution for dermal application.
The other components are :
Propylene glycol, ethanol 96%, perfume PCV 1676 (contains in particular butylhydroxytoluene).
Propellant gas: nitrogen.
Pharmaceutical form and content
This medication is in the form of a solution for skin application. Bottle of 30 g.
Name and address of the marketing authorization holder and the holder of the manufacturing authorization responsible for the release of the lots, if different
Holder
MYLAN SAS
117, allee des Parcs
69800 SAINT PRIEST
exploiting
MYLAN SAS
117, allee des Parcs
69800 SAINT PRIEST
Maker
Sandoz
49 avenue Georges Pompidou
92593 LEVALLOIS-PERRET CEDEX
or
CHEMINEAU LABORATORIES
93 Route de Monnaie
37210 VOUVRAY
Names of the medicinal product in the Member States of the European Economic Area
Not applicable.
Date of approval of the notice
The last date on which this leaflet was approved is {date}.
AMM under exceptional circumstances
Not applicable.
Internet Information
Detailed information on this medicine is available on the Afssaps website (France).
Information for health professionals only
Not applicable.
Other
Not applicable.