NOTICE
ANSM - Updated on: 16/02/2015
Name of the medicinal product
ONYTEC 80 mg / g, medicated nail varnish
ciclopirox
framed
Please read this leaflet carefully before you start using this medicine because it contains important information for you.
· Keep this leaflet, you may need to read it again.
· If you have any further questions, ask your doctor or pharmacist.
· If symptoms worsen or persist beyond 6 months for finger nails or 12 months for toes, consult your doctor.
· If you experience any side effects, talk to your doctor or pharmacist. This also applies to any side effects not mentioned in this leaflet. See section 4.
Review summary
In this notice :
1. WHAT ONYTEC 80 mg / g, medicated nail varnish AND IN WHAT CASES IS IT USED?
2. BEFORE YOU USE ONYTEC 80 mg / g, medicated nail varnish?
3. HOW TO USE ONYTEC 80 mg / g, medicated nail varnish?
4. WHAT ARE POSSIBLE SIDE EFFECTS?
5. HOW TO STORE ONYTEC 80 mg / g, medicated nail varnish?
6. ADDITIONAL INFORMATION
1. WHAT ONYTEC 80 mg / g, medicated nail varnish AND IN WHAT CASES IS IT USED?
Pharmacotherapeutic group
Pharmacotherapeutic group: Other antifungal agents for topical use
Therapeutic indications
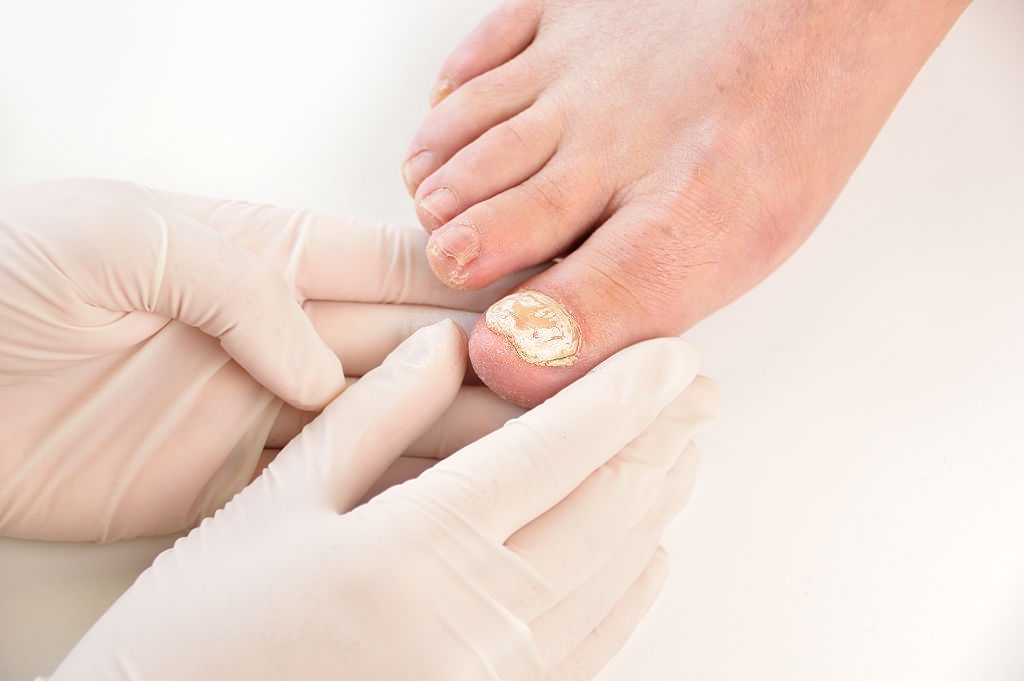
Nail polish ONY TEC 80 mg / g is a highly active antifungal agent (broad-spectrum antifungal agent), used locally on finger or toenail nails and on adjacent skin.
It is indicated for the treatment of light to moderate nail fungus (onychomycosis) due to filamentous fungi and / or other fungi that can be cured by ciclopirox.
The active substance, ciclopirox, is opposed to the growth of fungi and destroys them. This results in an improvement in the appearance of your nails.
2. BEFORE YOU USE ONYTEC 80 mg / g, medicated nail varnish?
List of information needed before taking the medication
Not applicable.
Cons-indications
Never use ONYTEC 80 mg / g, medicated nail varnish:
· if you are allergic (hypersensitive) to ciclopirox or any of the other ingredients of ONYTEC 80 mg / g, nail varnish,
· in children under the age of 18, due to lack of data in this age group.
Precautions for use; special warnings
Take special care with ONYTEC 80 mg / g, medicated nail varnish:
In case of sensitization, please stop treatment and consult a doctor.
As with any topical treatment of onychomycosis, in the case of involvement of more than one nail (more than 5), if more than two-thirds of the nail plate is reached and in the case of predisposing factors such as diabetes and the immune disorders, consult your doctor who could possibly add an oral treatment in addition to your medicated varnish.
If you are diabetic, be careful when cutting your nails.
Avoid contact with eyes and mucous membranes.
ONY TEC 80 mg / g, medicated nail varnish should only be used locally.
Do not apply ordinary nail varnish or other nail cosmetics to treated nails.
Interaction with other medicines
Use of other medicines
If you are taking or have recently taken any other medicines, including medicines obtained without a prescription, talk to your doctor or pharmacist.
Interactions with food and beverages
Not applicable.
Interactions with Herbal Medicines or Alternative Therapies
Not applicable.
Use during pregnancy and lactation
Pregnancy
Ask your doctor or pharmacist for advice before taking any medicine.
Treatment with ONYTEC 80 mg / g, nail varnish should only be initiated when absolutely necessary, after your doctor has carefully evaluated the benefits and possible risks.
feeding
There are no data on the passage of ciclopirox into breast milk in women. Treatment with ONYTEC 80 mg / g, nail varnish should only be initiated when absolutely necessary, after your doctor has carefully evaluated the benefits and possible risks.
Sport
Not applicable.
Effects on ability to drive or use machines
Not applicable.
List of excipients with known effect
Important information about some of the ingredients of ONYTEC 80 mg / g, medicated nail varnish:
ONYTEC 80 mg / g, nail polish contains cetostearyl alcohol. This can cause local skin reactions, such as redness and / or burning (irritative contact dermatitis).
3. HOW TO USE ONYTEC 80 mg / g, medicated nail varnish?
Instructions for proper use
Not applicable.
Dosage, Mode and / or route (s) of administration, Frequency of administration and Duration of treatment
Dosage
You should always use ONYTEC 80 mg / g, nail polish exactly as described in this leaflet. If in doubt, consult your doctor or pharmacist.
Administration mode
Unless otherwise prescribed by the doctor, ONYTEC 80 mg / g, nail polish should be applied in a thin layer, once a day, on the infected nail (s). For this, the nails must be clean and dry. The medicated nail varnish should be applied over the entire surface of the nail and over the 5 mm of adjacent skin. Where possible, ONYTEC 80 mg / g, nail polish should be applied under the free edge of the nail.
Let dry ONYTEC 80 mg / g, nail varnish for about thirty seconds.
Frequency of Administration
The nail (s) should not be cleaned for at least 6 hours and it is therefore recommended to apply it at night before bedtime. After this period, it is possible to carry out the usual hygiene care.
It is not necessary to remove ONYTEC 80 mg / g, nail varnish with a solvent or abrasive (eg a nail file); but the nails should be thoroughly cleaned with water. Sometimes due to insufficient washing of the nails, a white layer may appear on the surface of the nails after a few days of treatment. A thorough washing with neutral soap and if necessary with a nail brush or a sponge will help remove it .
Duration of treatment
Treatment should be continued until the problem is resolved, so that the nail (s) is free of any lesions and / or healthy nails have been repelled. Complete nail healing is normally achieved in about six months, that of the toenails in 9 to 12 months.
If the nail is severely affected by a single finger or toe or by several nails, an additional oral treatment may be considered. Please ask your doctor for advice if this is the case.
If the effect of ONYTEC 80 mg / g, nail varnish appears excessive or insufficient, talk to your doctor or pharmacist.
Symptoms and Instructions for Overdose
If you used more than ONYTEC 80 mg / g, medicated nail varnish you should:
No cases of overdose have been reported to date.
Instructions for omission of one or more doses
If you forget to use ONYTEC 80 mg / g, medicated nail varnish:
Do not use a double dose to compensate for the one you forgot to apply. Continue the treatment as prescribed by your doctor or in accordance with point 3 of this leaflet (How to use ONYTEC 80 mg / g, medicated nail varnish?). If you have not applied the nail polish for several days, its effectiveness may be reduced.
Risk of withdrawal syndrome
If you stop using ONYTEC 80 mg / g, medicated nail varnish:
If you stop ONYTEC 80 mg / g, nail varnish prior to complete or almost complete cure of your nails, it is possible that the fungus has not been eliminated. In this case, it is possible that the condition of your nails deteriorates again.
If you have any further questions on the use of this medication, ask your doctor or pharmacist.
4. WHAT ARE POSSIBLE SIDE EFFECTS?
Description of adverse reactions
Like all medicines, this medicine may cause side effects, although not everybody gets them.
Very rare side effects (affects less than 1 in 10,000 users):
Redness, flaking, burning and pruritus at the site of application.
Undesirable effects for which the frequency is indeterminate:
At the application site: rash, eczema
Transient fade of the nail (this reaction may also be due to the condition caused by nail fungus).
The adverse reactions reported were of mild intensity and short duration.
Declaration of side effects
If you experience any side effects, talk to your doctor or pharmacist. This also applies to any side effects not mentioned in this leaflet. You can also report adverse reactions directly through the national reporting system : National Agency for the Safety of Medicines and Health Products (Ansm) and network of Regional Centers of Pharmacovigilance. Website: www.ansm.sante.fr .
By reporting adverse reactions, you are helping to provide more information about the safety of the drug.
5. HOW TO STORE ONYTEC 80 mg / g, medicated nail varnish?
Keep out of the reach and sight of children.
Expiration date
Do not use the product after the expiry date which is stated on the carton and on the bottle.
Storage conditions
Keep the vial in the outer carton in order to protect from light.
Keep the bottle carefully closed to avoid evaporation of its contents.
Do not refrigerate.
This product is flammable, so keep it away from sources of heat or free flame.
After opening the bottle for the first time: keep at least 6 months.
If necessary, warnings against visible signs of deterioration
At temperatures below 15 ° C, nail polish may gel; a slight flocculation or the formation of a clear precipitate at the bottom of the flask is possible. It is possible to make them disappear by rubbing the bottle between your hands for about 1 minute. After this, the solution is again clear. These anomalies have no impact on the quality or effectiveness of the product.
Before use, visually inspect the bottle from below to verify that the solution is clear again (clear).
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist what to do with unused medications. These measures will help protect the environment.
6. ADDITIONAL INFORMATION
Full list of active substances and excipients
What contains ONYTEC 80 mg / g, medicated nail varnish?
The active substance is: ciclopirox.
One gram of the medicated nail varnish contains 80 mg of ciclopirox.
The other components are:
Ethyl acetate, 96% ethanol, cetostearyl alcohol, hydroxypropyl chitosan and purified water
Pharmaceutical form and content
What is ONYTEC 80 mg / g, medicated nail varnish and contents of the pack?
ONYTE C 80 mg / g, nail polish is a clear, colorless to slightly yellowish solution, presented in clear glass vials with a screw cap with an applicator brush.
Presentations of 3.3 ml and 6.6 ml.
Not all pack sizes may be marketed.
Name and address of the marketing authorization holder and the holder of the manufacturing authorization responsible for the release of the lots, if different
Holder
BAILLEUL LABORATORIES
8 rue LAUGIER
75017 PARIS
exploiting
BAILLEUL-BIORGA LABORATORIES
8, RUE LAUGIER
75017 PARIS
Maker
ALFA WASSERMANN SPA
VIA ENRICO FERMI N ° 1
65020 ALANNO (PE)
ITALY
or
DOPPEL FARMACEUTICI SRL
VIA MARTIRI DELLE FOIBE NO 1
29016 CORTEMAGGIORE (PC)
ITALY
Names of the medicinal product in the Member States of the European Economic Area
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
In accordance with the regulations.
Date of approval of the notice
The last date on which this leaflet was approved is {date}.
AMM under exceptional circumstances
Not applicable.
Internet Information
Detailed information on this medicine is available on the Ansm website (France).
Information for health professionals only
Not applicable.
Other
Not applicable.